The next generation of passive solar for cold climates

Photo courtesy Fred and Linda Driedger
Transforming energy involves a high amount of waste
The first law of thermodynamics states energy cannot be created or destroyed, although it can be transformed from one form to another. When energy is transformed, there are always inefficiencies—usually in the form of escaped heat. In fact, most energy systems involve inefficient transformations of energy. Photosynthesis, for instance, transforms light into chemical energy, yet around five per cent of the solar energy received is actually converted into energy for the plant, which compensates for this low efficiency by increasing the surface area of collection to survive. (This information comes from Photosynthesis by David O. Hall and Krishna Rao, published by Cambridge University Press in 1999.) It is amazing that with such low efficiency, photosynthesis can support the Earth’s food chain. Another example is the combustion engine—approximately 80 per cent of the energy released in the typical auto gasoline combustion process ends up as waste heat, while the remaining 20 per cent is used to power the vehicle.
PV technologies have been steadily improving, but the numbers are still lacking. The average solar cell converts around 15 per cent of sunlight into electricity. Therefore, if 1000 watts of sunlight (i.e. a clear summer day in Toronto) are received on a 1-m2 (11-sf) solar panel, only 150 watts get to the battery system or grid. Further losses occur in the transformation of the electricity into potential energy within the battery, and in the process of extracting the energy from the battery. As with the plant, the surface area of collection must be substantial to make the system feasible.
Typically, off-grid PV systems with batteries are not used for heating. Heating simply requires a massive battery system. A typical 1000-watt space heater, using the solar array listed above would need just under 7-m2 (75-sf) of solar panels in cloudless summer sun to make it work. As winter months tend to be very cloudy with limited daylight hours, even with battery systems, it just does not make sense at this time to use PV systems for heating.
Images courtesy Mark Driedger
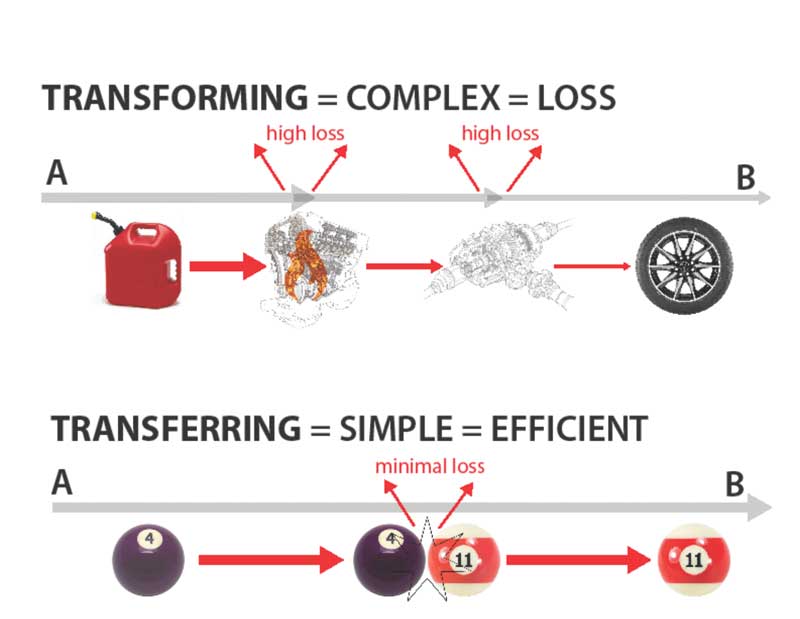
Transferring energy is simple and efficient
We can take a cue from nature for storing thermal energy. For instance, large bodies of water typically moderate the temperatures of adjacent areas. Ocean and lake water warms surrounding areas in the winter, while cooling the air in summer. The water captures the radiation from the sun as heat, keeping energy in its original state. Similarly, a black latex paint wall exposed to the sun will convert 96 per cent of the sun’s energy directly into heat. There is very little waste in simple transference (Figure 2) In 2011, with the help of Lawrence Technological University (LTU) in Detroit, this author took another look at what caused the A-frame solar project to become the family treehouse, with the goal of transferring rather than transforming solar to heat energy.
The weaknesses of the Falcon were addressed in the LTU experiment in the following manner:
Increasing the energy available to the system
By increasing the amount of sunlight hitting the thermal mass with multiple heliostats (i.e. reflectors), more energy can be made available for the system. As an example, a plant uses multiple leaves to maximize its receiving area—the system brings the concentrated sunlight directly into the insulated thermal mass rather than exposing the conditioned water to the exterior environment, as would happen in a typical solar hot water system.