Accelerated corrosion testing
Historic problems
Through the years, various challenges to the applicability of salt spray test data have been made. Clearly, many field applications do not involve exposure to salt chemicals, and rarely at a concentration level of five per cent. How meaningful, therefore, can salt fog data be?
For example, galvanized steel experiences a higher rate of corrosion in sulphide atmospheres compared to sulphide-free atmospheres, and corrosion reactions will not be the same in a chloride atmosphere as in a sulphide atmosphere. Therefore, salt spray test results would not be expected to correlate with outdoor performance in sulphide environments. Additionally, manufacturers do not recommend the use of coated steel sheets for applications involving continuous exposure to moisture (as occurs in the salt spray test).
In fact, the good performance of zinc-based coatings on steel requires drying between periods of wetness, and the need for these wet/dry cycles is generally well known. It is the development of a passive and relatively stable oxide and/or carbonate film during the drying cycle that contributes to the good performance of galvanized coatings. The continual wetness during the salt spray test does not allow this passive oxide/carbonate layer to develop.
When painted material is evaluated using the salt spray test, there is no exposure to ultraviolet (UV) light—a common cause of deterioration for paints and primers. This is a serious omission, since the failure mechanisms that eventually cause painted steel sheet to deteriorate are typically not included as conditions in the salt spray test.

Photo courtesy Teck Resources Limited
There are other vagaries that often appear in the salt spray test. For example, sample-to-sample variability for supposedly identical samples has been large. The test data gathered in two different cabinets has also been variable, even though the cabinets are identical in design and operation.
There are many reasons that the salt spray test does not correlate with most real-world exposure conditions. They include:
- the surface of the test coupons is constantly wet, with no cyclic drying, which does not happen in the field;
- the test chamber temperature is at a constant elevated 35 C (95 F), which increases water, oxygen, and ion transport compared to what would be experienced in the field; and
- the chloride content is very high at five per cent, preventing zinc from forming a passive film as it does in the field.
These are unusual and severe conditions that rarely, if ever, occur during natural weathering.
Does the test have any value?
There are those in the scientific community claiming the test has limited or no value. One can make a solid argument for this conclusion. Certainly, the practice of using the salt spray test to rank the relative performance of different coatings and/or paints is meaningless with respect to service performance. However, because there is so much historical data there are some general ways in which the test has value.
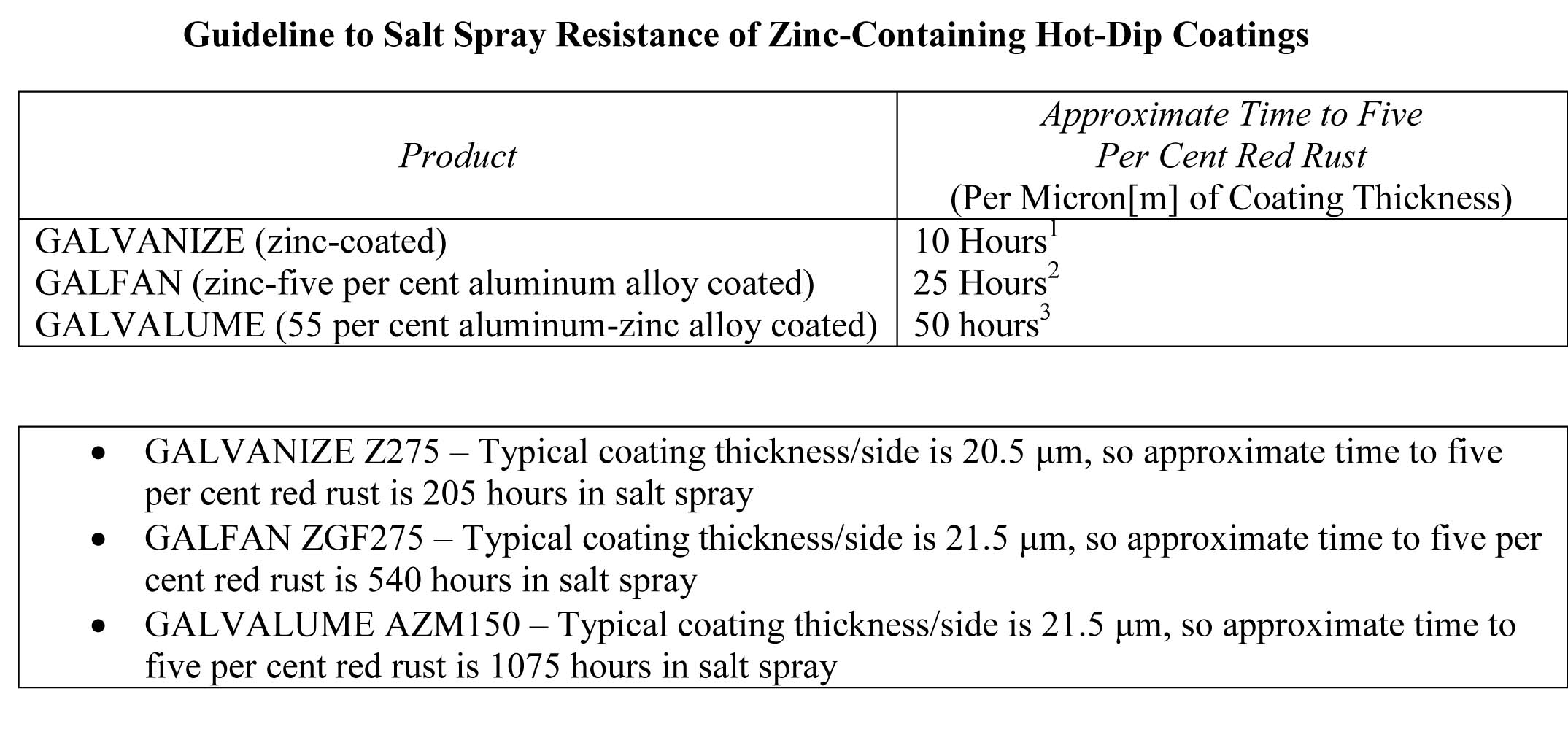
The performance of galvanized coatings on steel provides a good example. The salt spray test shows a linear relation between the thickness of the coating and its life (such as time to first rust). This is similar to the performance correlation in real world exposures. In most types of environmental exposure, the coating life is linear (i.e. twice the zinc coating thickness provides twice the product life). Although the salt spray test does not correlate with outdoor exposure sufficiently to claim a specific number of hours in the salt spray test will provide ‘x’ number of years life in a real-world application, it can be used to confirm a specific lot of material has approximately the coating thickness claimed by the seller. For example, if the life to 10 per cent rust is only 40 hours, it is essentially certain the coating does not meet the thickness requirements of the most commonly used G60 and G90 coatings.

Photo © Bigstock.com
To show the test’s value in another way, one can consider the performance of painted galvanized panels. The benefit of having a thick galvanized coating beneath the paint coating can be shown in the salt fog test. For example, when comparing a thin electroplated zinc-coating to a thicker G90 hot-dip galvanized coating, the salt spray testing will show the thin zinc coated sample with considerably more paint undercutting corrosion along a sheared edge of the panel compared to a painted G90 panel. This result means a thicker zinc coating is preferable beneath a paint coating for applications where high rates of corrosion are expected (e.g. outdoor roofing). Indeed, the value of a thicker zinc coating has been clearly demonstrated for applications where the corrosivity of an application is severe. Through the years, there have been numerous misapplications of painted galvanized steel sheet where the zinc-coating thickness was not sufficient to provide the service life expected by the user.
The salt spray test can be used to demonstrate the benefit of using a thicker galvanized coating to improve the product life in the field. However, these are qualitative evaluations. The limitation is using a thicker zinc coating to reduce the rate of paint undercutting corrosion along a sheared edge by one-half in the salt spray test in no way means the same reduction in undercutting corrosion will be observed in real-world applications.
The salt spray test can also be useful as a quality control test for painted steels. If a well-applied paint system (i.e. pretreatment, primer, and topcoat) has been shown to perform well in service, the periodic sampling of production materials has merit. For example, if the normal performance in the salt spray test is 500 hours before the onset of a specific amount of undercutting corrosion, the periodic testing of production lot samples is a quick way to determine whether there are any major production problems affecting the product quality. The salt spray test may not show conclusively the product quality is acceptable, but if the performance in this test is substandard, the outdoor performance may also be diminished. In this instance, a lack of proper quality control might be indicated.
There may be some natural environments where the salt spray test may more closely correlate qualitatively with results found in the field. One such environment is the splash zone of seacoasts. Another value of the test is it is severe—candidate materials may be able to be eliminated earlier in the selection process and perhaps save the expense of field testing.
Conclusion
Today, the salt spray test is so deeply embedded in the mindset of many users of coated steel sheet products that its elimination as a test procedure seems impossible. There are two primary reasons for this.
First, compliance with the salt spray test is contained in many industry and customer specifications in almost all consuming industries. Additionally, many of the companies who use these specifications make claims in their own product literature about the salt spray test ‘corrosion life’ of their products.
Second, there is no one universal accelerated corrosion test to replace the salt spray test. If the steel industry, the paint industry, and the treatment suppliers really desire to replace it, they need an easy to use alternative. As of today, no such alternative test exists. Several cyclic tests have been developed specifically for the automotive and prepainted building panel industries, but they have not been widely accepted as a replacement for salt fog testing. It may be too simplistic to expect any one accelerated corrosion test will correlate with all types of applications. If the user community is knowledgeable about what the salt spray test really means, they can then understand its limitations and use the results in a judicious manner.
 Gary Dallin, P. Eng. is director of the GalvInfo Center, a program of the International Zinc Association. Previously, he was employed as a metallurgist in the Canadian steel industry. Dallin has 47 years of experience with galvanized and prepainted steel sheet products, is a registered Professional Engineer in Ontario, and is active on ASTM International Committee A05 on metallic-coated steel products. Dallin can be contacted at info@galvinfo.com.
Gary Dallin, P. Eng. is director of the GalvInfo Center, a program of the International Zinc Association. Previously, he was employed as a metallurgist in the Canadian steel industry. Dallin has 47 years of experience with galvanized and prepainted steel sheet products, is a registered Professional Engineer in Ontario, and is active on ASTM International Committee A05 on metallic-coated steel products. Dallin can be contacted at info@galvinfo.com.